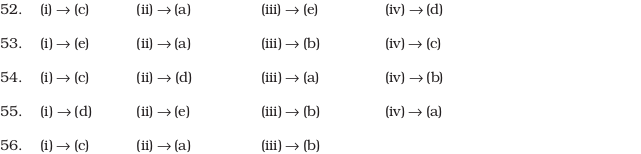NCERT Exemplar Class 11 Chemistry Chapter 4: Chemical Bonding and Molecular Structure. NCERT Exemplar Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure prepare students for their Class 11 exams thoroughly.
Chemistry problems and solutions for the Class 11 pdf are provided here which are similar to the questions being asked in the previous year’s board.
Contents
NCERT Exemplar Class 11 Chemistry Chapter 4: Chemical Bonding and Molecular Structure
Class 11: Chemistry Chapter 4 solutions. Complete Class 11 Chemistry Chapter 4 Notes.
Multiple Choice Questions (Type-I)
- Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs.
- (i) [NF3 and BF3]
- (ii) [BF4– and NH4+]
- (iii) [BCl3 and BrCl3]
- (iv) [NH3 and NO3–]
- Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
- (i) CO2
- (ii) HI
- (iii) H2O
- (iv) SO2
- The types of hybrid orbitals of nitrogen in NO2+, NO3– and NH4+ respectively are expected to be
- (i) sp, sp3 and sp2
- (ii) sp, sp2 and sp3
- (iii) sp2, sp and sp3
- (iv) sp2, sp3 and sp
- Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
- (i) HF > H2O > NH3
- (ii) H2O > HF > NH3
- (iii) NH3 > HF > H2O
- (iv) NH3 > H2O > HF
- In PO43– ion the formal charge on the oxygen atom of P–O bond is
- (i) + 1
- (ii) – 1
- (iii) – 0.75
- (iv) + 0.75
- In NO3– ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
- (i) 2, 2
- (ii) 3, 1
- (iii) 1, 3
- (iv) 4, 0
- Which of the following species has tetrahedral geometry?
- (i) BH4–
- (ii) NH2–
- (iii) CO32–
- (iv) H3O+
- Number of π bonds and σ bonds in the following structure is–

- (i) 6, 19
- (ii) 4, 20
- (iii) 5, 19
- (iv) 5, 20
- Which molecule/ion out of the following does not contain unpaired electrons?
- (i) N2+
- (ii) O2
- (iii) O22–
- (iv) B2
- In which of the following molecule/ion all the bonds are not equal?
- (i) XeF4
- (ii) BF4–
- (iii) C2H4
- (iv) SiF4
- In which of the following substances will hydrogen bond be strongest?
- (i) HCl
- (ii) H2O
- (iii) HI
- (iv) H2S
- If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in chemical bond formation will be_____.
- (i) 3p6
- (ii) 3p6, 4s2
- (iii) 3p6, 3d2
- (iv) 3d2, 4s2
- Which of the following angle corresponds to sp2 hybridisation?
- (i) 90°
- (ii) 120°
- (iii) 180°
- (iv) 109°

- Stable form of A may be represented by the formula :
- (i) A
- (ii) A2
- (iii) A3
- (iv) A4
- Stable form of C may be represented by the formula :
- (i) C
- (ii) C2
- (iii) C3
- (iv) C4
- The molecular formula of the compound formed from B and C will be
- (i) BC
- (ii) B2C
- (iii) BC2
- (iv) BC3
- The bond between B and C will be
- (i) Ionic
- (ii) Covalent
- (iii) Hydrogen
- (iv) Coordinate
- Which of the following order of energies of molecular orbitals of N2 is correct?
- (i) (π2py ) < (σ2pz) < (π*2px) ≈ (π*2py )
- (ii) (π2py ) > (σ2pz) > (π*2px) ≈ (π*2py )
- (iii) (π2py ) < (σ2pz) > (π*2px) ≈ (π*2py )
- (iv) (π2py ) > (σ2pz) < (π*2px) ≈ (π*2py)
- Which of the following statement is not correct from the view point of molecular orbital theory?
- (i) Be2 is not a stable molecule.
- (ii) He2 is not stable but He2+ is expected to exist.
- (iii) Bond strength of N2 is maximum amongst the homonuclear diatomic molecules belonging to the second period.
- (iv) The order of energies of molecular orbitals in N2 molecule is
σ2s < σ* 2s < σ2pz < (π2pX = π2pY) < (π*2pX = π*2pY) < σ*2pz
- Which of the following options represents the correct bond order :
- (i) O2– > O2 > O2+
- (ii) O2– < O2 < O2+
- (iii) O2– > O2 < O2+
- (iv) O2– < O2 > O2+
- The electronic configuration of the outer most shell of the most electronegative element is
- (i) 2s22p5
- (ii) 3s23p5
- (iii) 4s24p5
- (iv) 5s25p5
- Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
- (i) [Ne]3s23p1
- (ii) [Ne]3s23p3
- (iii) [Ne]3s23p2
- (iv) [Ar]3d104s24p3
Multiple Choice Questions (Type-II)
In the following questions two or more options may be correct.
- Which of the following have identical bond order?
- (i) CN–
- (ii) NO+
- (iii) O2–
- (iv) O22-
- Which of the following attain the linear structure:
- (i) BeCl2
- (ii) NCO+
- (iii) NO2
- (iv) CS2
- CO is isoelectronic with
- (i) NO+
- (ii) N2
- (iii) SnCl2
- (iv) NO2–
- Which of the following species have the same shape?
- (i) CO2
- (ii) CCl4
- (iii) O3
- (iv) NO2–
- Which of the following statements are correct about CO32–?
- (i) The hybridisation of central atom is sp3.
- (ii) Its resonance structure has one C–O single bond and two C=O double bonds.
- (iii) The average formal charge on each oxygen atom is 0.67 units.
- (iv) All C–O bond lengths are equal.
- Dimagnetic species are those which contain no unpaired electrons. Which among the following are dimagnetic?
- (i) N2
- (ii) N22–
- (iii) O2
- (iv) O22–
- Species having same bond order are :
- (i) N2
- (ii) N2–
- (iii) F2+
- (iv) O2–
- Which of the following statements are not correct?
- (i) NaCl being an ionic compound is a good conductor of electricity in the solid state.
- (ii) In canonical structures there is a difference in the arrangement of atoms.
- (iii) Hybrid orbitals form stronger bonds than pure orbitals.
- (iv) VSEPR Theory can explain the square planar geometry of XeF4.
Short Answer Type Questions
- Explain the non linear shape of H2S and non planar shape of PCl3 using valence shell electron pair repulsion theory.
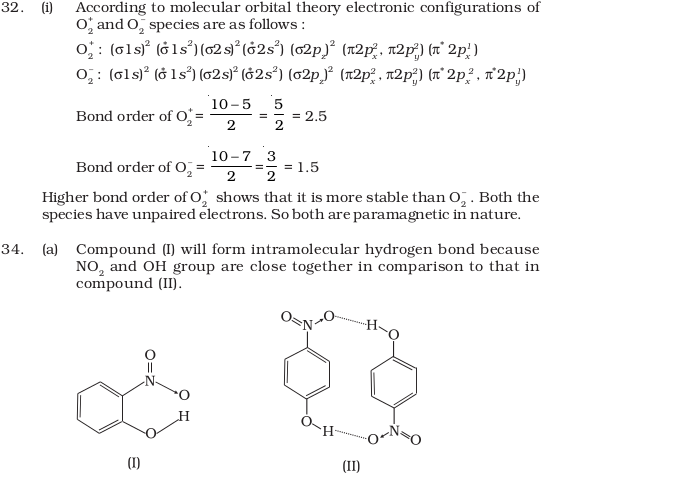
- Using molecular orbital theory, compare the bond energy and magnetic character of O2+ and O2– species.
- Explain the shape of BrF5.
- Structures of molecules of two compounds are given below :

- (a) Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding.
- (b) The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis explain which of the above two compounds will show higher melting point.
- (c) Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it.
- Why does type of overlap given in the following figure not result in bond formation?

- Explain why PCl5 is trigonal bipyramidal whereas IF5 is square pyramidal.
- In both water and dimethyl ether (CH3 — Ö — CH3), oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles.
Which one has greater bond angle? Give reason. - Write Lewis structure of the following compounds and show formal charge on each atom.
HNO3, NO2, H2SO4 - The energy of σ2pz molecular orbital is greater than π2px and π2py molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species :
N2, N2+>/sup> , N2– , N22+ - What is the effect of the following processes on the bond order in N2 and O2?
- (i) N2 → N2+ + e–
- (ii) O2 → O2+ + e–
- Give reasons for the following :
- (i) Covalent bonds are directional bonds while ionic bonds are nondirectional.
- (ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
- (iii) Ethyne molecule is linear.
- What is an ionic bond? With two suitable examples explain the difference between an ionic and a covalent bond?
- Arrange the following bonds in order of increasing ionic character giving reason.
N—H, F—H, C—H and O—H - Explain why CO32– ion cannot be represented by a single Lewis structure. How can it be best represented?
- Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.

- Group the following as linear and non-linear molecules :
H2O, HOCl, BeCl2, Cl2O - Elements X, Y and Z have 4, 5 and 7 valence electrons respectively.
- (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen.
- (ii) Which of these compounds will have the highest dipole moment?
- Draw the resonating structure of
- (i) Ozone molecule
- (ii) Nitrate ion
- Predict the shapes of the following molecules on the basis of hybridisation.
BCl3, CH4, CO2, NH3 - All the C—O bonds in carbonate ion (CO32–) are equal in length. Explain.
- What is meant by the term average bond enthalpy? Why is there difference in bond enthalpy of O—H bond in ethanol (C2H5OH) and water?
Matching Type Questions
- Match the species in Column I with the type of hybrid orbitals in Column II.

- Match the species in Column I with the geometry/shape in Column II.

- Match the species in Column I with the bond order in Column II.

- Match the items given in Column I with examples given in Column II.

- Match the shape of molecules in Column I with the type of hybridisation in Column II.

Assertion and Reason Type Questions
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
- Assertion (A) : Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason (R) : This is because sodium and chloride ions acquire octet in sodium chloride formation.- (i) A and R both are correct, and R is the correct explanation of A.
- (ii) A and R both are correct, but R is not the correct explanation of A.
- (iii) A is true but R is false.
- (iv) A and R both are false.
- Assertion (A) : Though the central atom of both NH3 and H2O molecules are sp3 hybridised, yet H–N–H bond angle is greater than that of H–O–H.
Reason (R) : This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.- (i) A and R both are correct, and R is the correct explanation of A.
- (ii) A and R both are correct, but R is not the correct explanation of A.
- (iii) A is true but R is false.
- (iv) A and R both are false.
- Assertion (A): Among the two O–H bonds in H2O molecule, the energy required to break the first O–H bond and the other O–H bond is the same.
Reason (R) : This is because the electronic environment around oxygen is the same even after breakage of one O–H bond.- (i) A and R both are correct, and R is correct explanation of A.
- (ii) A and R both are correct, but R is not the correct explanation of A.
- (iii) A is true but R is false.
- (iv) A and R both are false.
Long Answer Type Questions
- i) Discuss the significance/ applications of dipole moment.
(ii) Represent diagrammatically the bond moments and the resultant dipole moment in CO2, NF3 and CHCl3. - Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2, a single bond and Ne2, no bond.
- Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
- Describe hybridisation in the case of PCl5 and SF6. The axial bonds are longer as compared to equatorial bonds in PCl5 whereas in SF6 both axial bonds and equatorial bonds have the same bond length. Explain.
- 64. (i) Discuss the concept of hybridisation. What are its different types in a carbon atom.
(ii) What is the type of hybridisation of carbon atoms marked with star.
Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option.
Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and anti bonding molecular orbital (ABMO). Energy of anti bonding orbital is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order :
Different atomic orbitals of one atom combine with those atomic orbitals of the second atom which have comparable energies and proper orientation. Further, if the overlapping is head on, the molecular orbital is called ‘Sigma’, (σ) and if the overlap is lateral, the molecular orbital is called ‘pi’, (π). The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all molecules or their ions. Bond order is one of the most important parameters to compare the strength of bonds. - Which of the following statements is correct?
- (i) In the formation of dioxygen from oxygen atoms 10 molecular orbitals will be formed.
- (ii) All the molecular orbitals in the dioxygen will be completely filled.
- (iii) Total number of bonding molecular orbitals will not be same as total number of anti bonding orbitals in dioxygen.
- (iv) Number of filled bonding orbitals will be same as number of filled antibonding orbitals.
- Which of the following molecular orbitals has maximum number of nodal planes?
- (i) σ*1s
- (ii) σ*2pz
- (iii) π2px
- (iv) π*2py
- Which of the following pair is expected to have the same bond order?
- (i) O2 , N2
- (ii) O2+, N2–
- (iii) O2 – , N2+
- (iv) O2–, N2–
- In which of the following molecules, σ2pz molecular orbital is filled after π2px and π2py molecular orbitals?
- (i) O2
- (ii) Ne2
- (iii) N2
- (iv) F2
Answers to Multiple Choice Questions
| MCQ (Type-I) | |||||||||||
| Q.No. | Answer | Q.No. | Answer | Q.No. | Answer | Q.No. | Answer | Q.No. | Answer | Q.No. | Answer |
| 1 | (ii) | 2 | (iii) | 3 | (ii) | 4 | (ii) | 5 | (ii) | 6 | (iv) |
| 7 | (i) | 8 | (iii) | 9 | (iii) | 10 | (iii) | 11 | (ii) | 12 | (iv) |
| 13 | (ii) | 14 | (i) | 15 | (ii) | 16 | (iv) | 17 | (ii) | 18 | (i) |
| 19 | (iv) | 20 | (ii) | 21 | (i) | 22 | (ii) |
| MCQ (Type-II) | |||||
| Q.No. | Answer | Q.No. | Answer | Q.No. | Answer |
| 1 | (i), (ii) | 2 | (i), (iv) | 3 | (i), (ii) |
| 4 | (iii), (iv) | 5 | (iii), (iv) | 6 | (i), (iv) |
| 7 | (iii), (iv) | 8 | (i), (ii) |