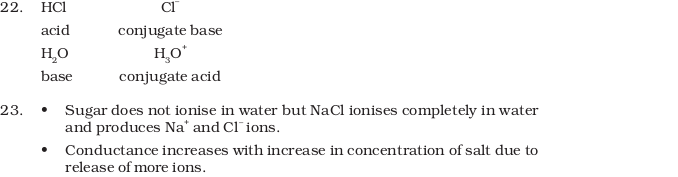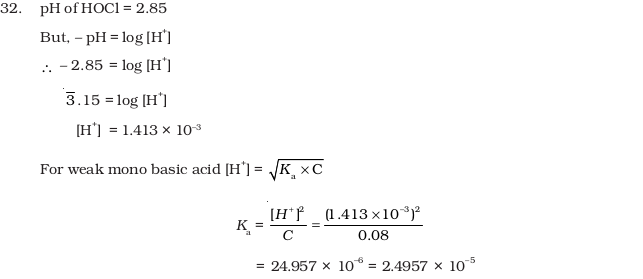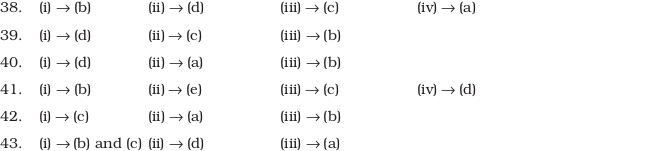NCERT Exemplar Class 11 Chemistry Chapter 7: Equilibrium. NCERT Exemplar Solutions for Class 11 Chemistry Chapter 7 Equilibrium prepare students for their Class 11 exams thoroughly.
Chemistry problems and solutions for the Class 11 pdf are provided here which are similar to the questions being asked in the previous year’s board.
Contents
NCERT Exemplar Class 11 Chemistry Chapter 7: Equilibrium
Class 11: Chemistry Chapter 7 solutions. Complete Class 11 Chemistry Chapter 7 Notes.
Multiple Choice Questions (Type-I)
- We know that the relationship between Kc and Kp is
Kp = Kc (RT)Δ n
What would be the value of Δn for the reaction
NH4Cl (s) ⇔ NH3 (g) + HCl (g)- (i) 1
- (ii) 0.5
- (iii) 1.5
- (iv) 2
- For the reaction H2(g) + I2(g) ⇔ 2HI (g), the standard free energy is ΔG⊖ > 0. The equilibrium constant (K ) would be __________.
- (i) K = 0
- (ii) K > 1
- (iii) K = 1
- (iv) K < 1
- Which of the following is not a general characteristic of equilibria involving physical processes?
- (i) Equilibrium is possible only in a closed system at a given temperature.
- (ii) All measurable properties of the system remain constant.
- (iii) All the physical processes stop at equilibrium.
- (iv) The opposing processes occur at the same rate and there is dynamic but stable condition.
- PCl5, PCl3 and Cl2 are at equilibrium at 500K in a closed container and their concentrations are 0.8 × 10–3 mol L–1, 1.2 × 10–3 mol L–1 and 1.2 × 10–3 mol L–1 respectively. The value of Kc for the reaction PCl5 (g) ⇔ PCl3 (g) + Cl2 (g) will be
- (i) 1.8 × 103 mol L–1
- (ii) 1.8 × 10–3
- (iii) 1.8 × 10–3 L mol–1
- (iv) 0.55 × 104
- Which of the following statements is incorrect?
- (i) In equilibrium mixture of ice and water kept in perfectly insulated flask mass of ice and water does not change with time.
- (ii) The intensity of red colour increases when oxalic acid is added to a solution containing iron (III) nitrate and potassium thiocyanate.
- (iii) On addition of catalyst the equilibrium constant value is not affected.
- (iv) Equilibrium constant for a reaction with negative ΔH value decreases as the temperature increases.
- When hydrochloric acid is added to cobalt nitrate solution at room temperature, the following reaction takes place and the reaction mixture becomes blue. On cooling the mixture it becomes pink. On the basis of this information mark the correct answer.

- (i) ΔH > 0 for the reaction
- (ii) ΔH < 0 for the reaction
- (iii) ΔH = 0 for the reaction
- (iv) The sign of ΔH cannot be predicted on the basis of this information.
- The pH of neutral water at 25°C is 7.0. As the temperature increases, ionisation of water increases, however, the concentration of H+ ions and OH– ions are equal. What will be the pH of pure water at 60°C?
- (i) Equal to 7.0
- (ii) Greater than 7.0
- (iii) Less than 7.0
- (iv) Equal to zero
- The ionisation constant of an acid, Ka, is the measure of strength of an acid. The Ka values of acetic acid, hypochlorous acid and formic acid are 1.74 × 10–5, 3.0 × 10–8 and 1.8 × 10–4 respectively. Which of the following orders of pH of 0.1 mol dm–3 solutions of these acids is correct?
- (i) acetic acid > hypochlorous acid > formic acid
- (ii) hypochlorous acid > acetic acid > formic acid
- (iii) formic acid > hypochlorous acid > acetic acid
- (iv) formic acid > acetic acid > hypochlorous acid
- Ka1, Ka2 and Ka3 are the respective ionisation constants for the following reactions.
H2S ⇔ H+ + HS–
HS– ⇔ H+ + S2–
H2S ⇔ 2H+ + S2–
The correct relationship between Ka1, Ka2 and Ka3 is- (i) Ka3 = Ka1 * Ka2
- (ii) Ka3 = Ka1 + Ka2
- (iii) Ka3 = Ka1 – Ka2
- (iv) Ka3 = Ka1 / Ka2
- Acidity of BF3 can be explained on the basis of which of the following concepts?
- (i) Arrhenius concept
- (ii) Bronsted Lowry concept
- (iii) Lewis concept
- (iv) Bronsted Lowry as well as Lewis concept.
- Which of the following will produce a buffer solution when mixed in equal volumes ?
- (i) 0.1 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
- (ii) 0.05 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
- (iii) 0.1 mol dm–3 NH4OH and 0.05 mol dm–3 HCl
- (iv) 0.1 mol dm–3 CH4COONa and 0.1 mol dm–3 NaOH
- In which of the following solvents is silver chloride most soluble?
- (i) 0.1 mol dm–3 AgNO3 solution
- (ii) 0.1 mol dm–3 HCl solution
- (iii) H2O
- (iv) Aqueous ammonia
- What will be the value of pH of 0.01 mol dm–3 CH3COOH (Ka = 1.74 × 10–5)?
- (i) 3.4
- (ii) 3.6
- (iii) 3.9
- (iv) 3.0
- Ka for CH3COOH is 1.8 × 10–5 and Kb for NH4OH is 1.8 × 10-5 . The pH of ammonium acetate will be
- (i) 7.005
- (ii) 4.75
- (iii) 7.0
- (iv) Between 6 and 7
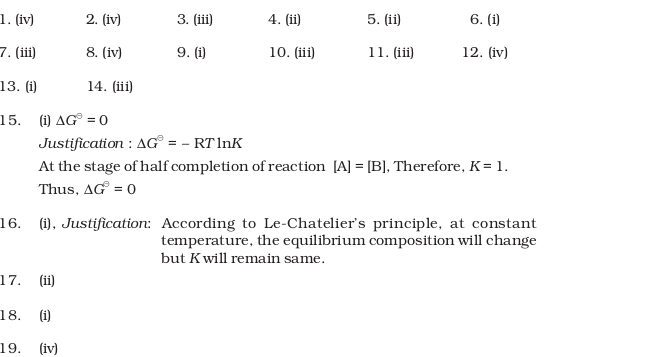
- Which of the following options will be correct for the stage of half completion of the reaction A ⇔ B.
- (i) ΔG⊖ = 0
- (ii) ΔG⊖ > 0
- (iii) ΔG⊖ < 0
- (iv) ΔG⊖ = –RT ln2
- On increasing the pressure, in which direction will the gas phase reaction proceed to re-establish equilibrium, is predicted by applying the Le Chatelier’s principle. Consider the reaction.
N2(g) + 3H2(g) ⇔ 2NH3(g)
Which of the following is correct, if the total pressure at which the equilibrium is established, is increased without changing the temperature?- (i) K will remain same
- (ii) K will decrease
- (iii) K will increase
- (iv) K will increase initially and decrease when pressure is very high
- What will be the correct order of vapour pressure of water, acetone and ether at 30°C. Given that among these compounds, water has maximum boiling point and ether has minimum boiling point?
- (i) Water < ether < acetone
- (ii) Water < acetone < ether
- (iii) Ether < acetone < water
- (iv) Acetone < ether < water
- At 500 K, equilibrium constant, Kc, for the following reaction is 5.
1 / 2 H2 (g) + 1 / 2 I2 (g) ⇔ HI (g)
What would be the equilibrium constant Kc for the reaction
2HI (g) ⇔ H2 (g) + I2 (g)- (i) 0.04
- (ii) 0.4
- (iii) 25
- (iv) 2.5
- In which of the following reactions, the equilibrium remains unaffected on addition of small amount of argon at constant volume?
- (i) H2 (g) + I2 (g) ⇔ 2HI (g)
- (ii) PCl5 (g) ⇔ PCl3 (g) + Cl2(g)
- (iii) N2(g) + 3H2 (g) ⇔ 2NH3 (g)
- (iv) The equilibrium will remain unaffected in all the three cases.
Multiple Choice Questions (Type-II)
In the following questions two or more options may be correct.
- For the reaction N2 O4 (g) ⇔ 2NO2 (g), the value of K is 50 at 400 K and 1700 at 500 K. Which of the following options is correct?
- (i) The reaction is endothermic
- (ii) The reaction is exothermic
- (iii) If NO2 (g) and N2 O4 (g) are mixed at 400 K at partial pressures 20 bar and 2 bar respectively, more N2 O4 (g) will be formed.
- (iv) The entropy of the system increases.
- At a particular temperature and atmospheric pressure, the solid and liquid phases of a pure substance can exist in equilibrium. Which of the following term defines this temperature?
- (i) Normal melting point
- (ii) Equilibrium temperature
- (iii) Boiling point
- (iv) Freezing point
Short Answer Type Questions
- The ionisation of hydrochloric in water is given below:
HCl(aq) + H2O (l ) ⇔ H3O+ (aq) + Cl– (aq)
Label two conjugate acid-base pairs in this ionisation. - The aqueous solution of sugar does not conduct electricity. However, when sodium chloride is added to water, it conducts electricity. How will you explain this statement on the basis of ionisation and how is it affected by concentration of sodium chloride?
- BF3 does not have proton but still acts as an acid and reacts with NH3. Why is it so? What type of bond is formed between the two?
- Ionisation constant of a weak base MOH, is given by the expression

Values of ionisation constant of some weak bases at a particular temperature are given below:
Arrange the bases in decreasing order of the extent of their ionisation at equilibrium. Which of the above base is the strongest? - Conjugate acid of a weak base is always stronger. What will be the decreasing order of basic strength of the following conjugate bases?
OH–,RO–, CH3COO–, Cl– - Arrange the following in increasing order of pH.
KNO3 (aq), CH3COONa (aq), NH4Cl (aq), C6H5COONH4(aq) - The value of Kc for the reaction 2HI (g) ⇔ H2 (g) + I2 (g) is 1 × 10-4
At a given time, the composition of reaction mixture is
[HI] = 2 × 10-5 mol, [H2] = 1 × 10-5 mol and [I2] = 1 × 10-5 mol
In which direction will the reaction proceed? - On the basis of the equation pH = – log [H+], the pH of 10-8 mol dm-3 solution of HCl should be 8. However, it is observed to be less than 7.0. Explain the reason.
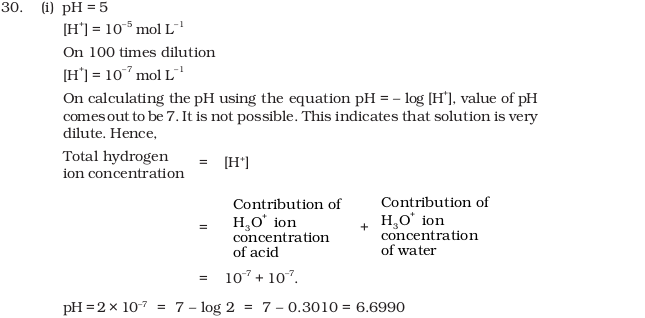
- pH of a solution of a strong acid is 5.0. What will be the pH of the solution obtained after diluting the given solution a 100 times?
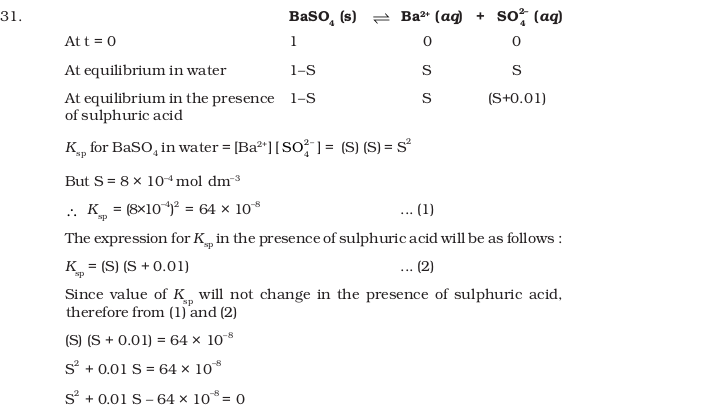
- A sparingly soluble salt gets precipitated only when the product of concentration of its ions in the solution (Qsp) becomes greater than its solubility product. If the solubility of BaSO4 in water is 8 × 10-4 mol dm-3. Calculate its solubility in 0.01 mol dm-3 of H2SO4.
- pH of 0.08 mol dm–3 HOCl solution is 2.85. Calculate its ionisation constant.
- Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.
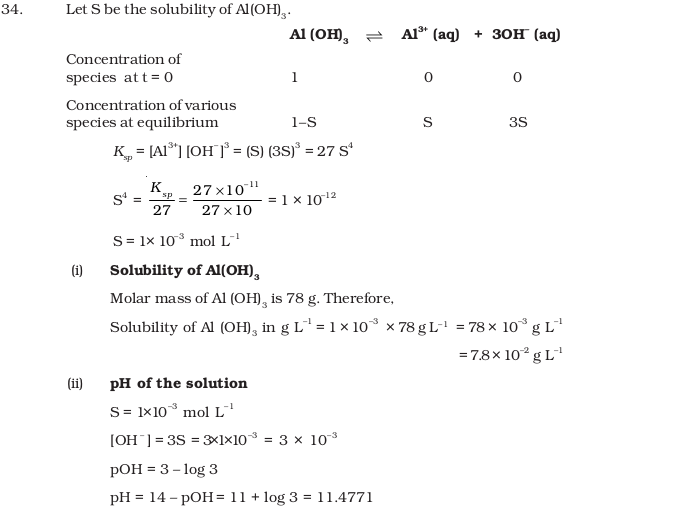
- The solubility product of Al (OH)3 is 2.7 × 10–11. Calculate its solubility in gL–1 and also find out pH of this solution. (Atomic mass of Al = 27 u).
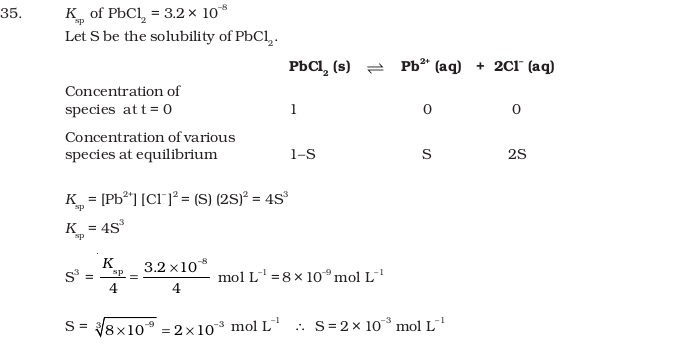
- Calculate the volume of water required to dissolve 0.1 g lead (II) chloride to get a saturated solution. (Ksp of PbCl2 = 3.2 × 10–8 , atomic mass of Pb = 207 u).
- A reaction between ammonia and boron trifluoride is given below:
: NH3 + BF3 → H3N : BF3
Identify the acid and base in this reaction. Which theory explains it? What is the hybridisation of B and N in the reactants? - Following data is given for the reaction: CaCO3(s) → CaO (s) + CO2(g)
Δf H⊖ [CaO(s)] = – 635.1 kJ mol–1
Δf H⊖ [CO2(g)] = – 393.5 kJ mol–1
Δf H⊖ [CaCO3(s)] = – 1206.9 kJ mol–1
Predict the effect of temperature on the equilibrium constant of the above reaction.
Matching Type Questions
- Match the following equilibria with the corresponding condition


Some reactions are written below in Column I and their equilibrium constants in terms of Kc are written in Column II. Match the following reactions with the corresponding equilibrium constant
- Match standard free energy of the reaction with the corresponding equilibrium constant

- Match the following species with the corresponding conjugate acid

- Match the following graphical variation with their description

- Match Column (I) with Column (II).

Assertion and Reason Type Questions
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
- Assertion (A) : Increasing order of acidity of hydrogen halides is HF < HCl < HBr < HI
Reason (R) : While comparing acids formed by the elements belonging to the same group of periodic table, H–A bond strength is a more important factor in determining acidity of an acid than the polar nature of the bond.- (i) Both A and R are true and R is the correct explanation of A.
- (ii) Both A and R are true but R is not the correct explanation of A.
- (iii) A is true but R is false.
- (iv) Both A and R are false.
- Assertion (A) : A solution containing a mixture of acetic acid and sodium acetate maintains a constant value of pH on addition of small amounts of acid or alkali.
Reason (R) : A solution containing a mixture of acetic acid and sodium acetate acts as a buffer solution around pH 4.75.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not the correct explanation of A.
- (iii) A is true but R is false.
- (iv) Both A and R are false.
- Assertion (A): The ionisation of hydrogen sulphide in water is low in the presence of hydrochloric acid.
Reason (R) : Hydrogen sulphide is a weak acid.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not correct explanation of A.
- (iii) A is true but R is false
- (iv) Both A and R are false
- Assertion (A): For any chemical reaction at a particular temperature, the equilibrium constant is fixed and is a characteristic property.
Reason (R) : Equilibrium constant is independent of temperature.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not correct explanation of A.
- (iii) A is true but R is false.
- (iv) Both A and R are false.
- Assertion (A) : Aqueous solution of ammonium carbonate is basic.
Reason (R) : Acidic/basic nature of a salt solution of a salt of weak acid and weak base depends on Ka and Kb value of the acid and the base forming it.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not correct explanation of A.
- (iii) A is true but R is false.
- (iv) Both A and R are false.
- Assertion (A): An aqueous solution of ammonium acetate can act as a buffer.
Reason (R) : Acetic acid is a weak acid and NH4OH is a weak base.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not correct explanation of A.
- (iii) A is false but R is true.
- (iv) Both A and R are false.
- Assertion (A): In the dissociation of PCl5 at constant pressure and temperature addition of helium at equilibrium increases the dissociation of PCl5.
Reason (R) : Helium removes Cl2 from the field of action.- (i) Both A and R are true and R is correct explanation of A.
- (ii) Both A and R are true but R is not correct explanation of A.
- (iii) A is true but R is false.
- (iv) Both A and R are false.
Long Answer Type Questions
- How can you predict the following stages of a reaction by comparing the value of Kc and Qc ?
- (i) Net reaction proceeds in the forward direction.
- (ii) Net reaction proceeds in the backward direction.
- (iii) No net reaction occurs.
- On the basis of Le Chatelier principle explain how temperature and pressure can be adjusted to increase the yield of ammonia in the following reaction.
N2(g) + 3H2(g) ⇔ 2NH3(g) Δ H = – 92.38 kJ mol–1
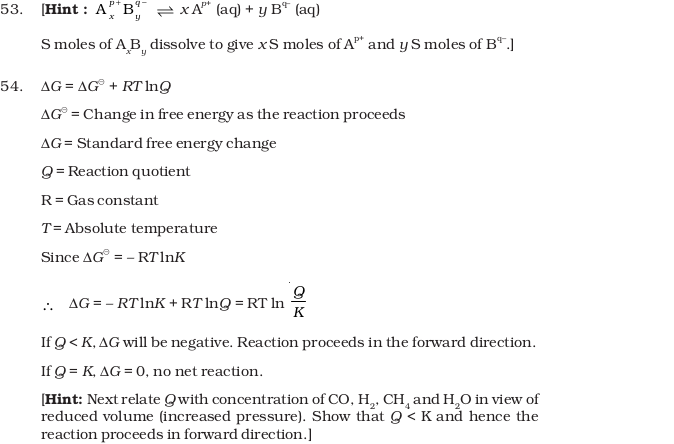
What will be the effect of addition of argon to the above reaction mixture at constant volume? - A sparingly soluble salt having general formula A p+x Bq-yand molar solubility S is in equilibrium with its saturated solution. Derive a relationship between the solubility and solubility product for such salt.
- Write a relation between ΔG and Q and define the meaning of each term and answer the following :
- (a) Why a reaction proceeds forward when Q < K and no net reaction occurs when Q = K.
- (b) Explain the effect of increase in pressure in terms of reaction quotient Q. for the reaction : CO (g) + 3H2(g) ⇔ CH4(g) + H2O (g)
Answers to Multiple Choice Questions
Answers to Multiple Choice Questions